Russia Medical Device Registration Process
I. Introduction of RZN
Federal Service for Surveillance in Healthcare (Roszdravnadzor), RZN
II. Regulations
Resolution No. 1416 "State Registration of Medical Devices" and its subsequent supplements.
Decree No. 2N "Technical Trials, Toxicological Studies and Clinical Trials".
Decree No. 4N "Medical Devices Nomenclature Classification".
Decree No. 7N "Import of Medical Products".
Decree No. 11N "Technical Files and Operation Manual".
III. Classification
Class I, IIa, IIb, III (risk level from low to high)
IV. RZN Certification Information
Certificate: CE, ISO, PoA, WIPO (Certificate of registered trademark in Russia), DoC;
Technical documents: STED, product description, test report (local physical, chemical and toxicological tests), IFU, labeling, raw material information, packaging information, validation reports, production process, color pages, biological reports, CER, clinical trial reports, RMR, physical drawings of the product and so on.
V. System Assessment
Phase I:
Check the technical documents to ensure the safety and validity of device-related information;
Inspection in terms of quality, efficiency and safety is carried out by an expert organization within a period of no more than 20 working days;
At the end of the inspection, a conclusion is drawn on the possibility of conducting clinical trials. (for medical devices with risk classes IIa, IIb and III)
Confirmation of clinical data:
Conducting clinical trials;
Submission of clinical data. (At the time of Phase I document entry, RZN informs of an on-site system audit)
Phase II:
Review of clinical data within a period not exceeding 10 working days;
At the end of the examination, a decision will be made whether to issue a certificate of registration.
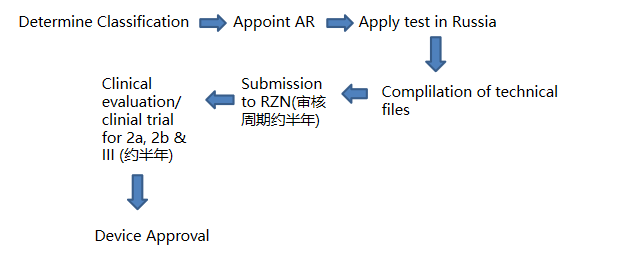
VI. RZN medical device registration process
1. Determine the classification to which the medical device product belongs and the classification code of the medical device according to the medical device naming and coding system;
2. Designate the authorized representative of Russia and sign the agreement;
3. The authorized representative of Russia applies for sample import license from RZN;
4. Carry out preclinical testing of the samples, including but not limited to: toxicology, safety, electromagnetic compatibility, etc.; (the testing organization must be a laboratory accredited by RZN)
5. Submit technical documentation to RZN by an authorised representative of Russia. The complete technical and descriptive documentation must include risk management documentation.