Medical Device Registration Process in Australia
I. TGA introduction
According to the Therapeutic Goods Act in Australia, all therapeutic goods (medicines and medical devices) marketed in Australia must be submitted for registration or listing with the Therapeutic Goods Administration (TGA) as per the relevant requirements to obtain legal market access and be included in the Australian Register of Therapeutic Goods (ARTG).
II. Applicable Regulations
1.Therapeutic Goods Act 1989-the Act;
2.Therapeutic Goods(Medical Devices)Regulations 2002-the Regulations。
III. Medical Device Classification
Australia is one of the few developed countries in the world that directly regulates “traditional Chinese medicine” and “health supplements” as “therapeutic goods.” It is also known for having clear procedures, well-defined standards, and a relatively straightforward path to successful registration. Through the Therapeutic Goods Administration (TGA) in Australia, for example:
Category | Risk level | Examples of medical devices |
Class I | Low | Surgical retractors and tongue depressors |
Class I - Sterility | Moderately Low | Subcutaneous injection needles and aspiration devices. |
Class I - Includes measurement function | Moderately Low | Medicine cups with specific measurement units. |
Class IIa | Moderately Low | Digital or infrared thermometers. |
Class IIb | Moderately High | Ventilators, blood bags, condoms. |
Class III | Heart valves, major joint replacement implants, devices containing drugs or tissues of animal, human, or microbial origin. | |
Active Implantable Medical Devices (AIMD) | High | Implantable defibrillator |
IV.TGA Certification information
①Australian Technical File (ATF): The applicant for a medical device in Australia needs to prepare an ATF, which includes technical specifications, performance data, clinical evaluation, and other relevant information.
②Safety and efficacy data
③Quality Management System Certification
④Labels and Instructions for Use
V. System assessment
1. TGA monitors medical devices, even if they have been approved for use in Australia, to ensure they continue to meet our safety standards and regulatory requirements. This is known as post-market surveillance. Some post-market monitoring activities include:
(1)Assessing and investigating medical device problem reports
(2)Evaluating whether medical devices continue to meet essential principles' evidence
(3)Conducting regular inspections of manufacturers
(4)Requiring manufacturers and sponsors to report adverse events and other information related to their medical devices within specific timeframes.
Reviewing adverse event reports is one way TGA monitors the safety of therapeutic goods used in Australia. TGA collects these reports into a database and regularly monitors them to identify spikes or unusual trends. A team of clinical doctors and scientists within TGA conducts risk assessments to determine whether investigations are necessary, and company may also seek expert advice. These investigations can lead to product recalls, safety alerts, modifications/improvements to the product by the manufacturer, or surveillance audits of manufacturing sites.
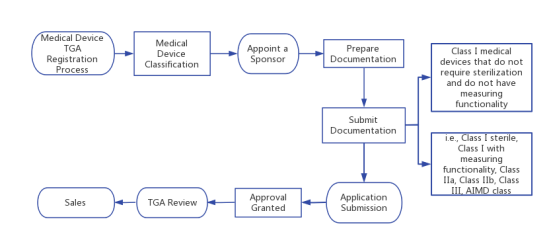
VI. TGA Registration Process of Medical Device
1. Medical Device Classification: The classification is in accordance with Schedule 2 of the Australian Therapeutic Goods (Medical Devices) Regulations 2002,. If your device has a European CE marking, the classification may be the same. As part of registration, TGA typically accepts CE marking certificate from notified body. TGA also accepts MDSAP certificate with acceptable overseas marketing approvals (e.g., Health Canada MDL, Japan MHLW/PMDA PMC or PMA, US FDA 510(k), or US FDA new PMA); or QMS certification from Japan MHLW/PMDA for Japan MHLW/PMDA PMC or PMA.
2. Appoint a Sponsor: If you do not have a local office in Australia, appoint an Australian TGA sponsor. A sponsor can facilitate the registration of your device, act as the point of contact between the manufacturer and the TGA, and the sponsor's name must appear on your device and labeling.
3. Prepare Documentation: Gather the latest technical files or design records and the Australian Declaration of Conformity that you need to submit.
4. Submit Documentation: For all devices other than Class I non-sterile, non-measuring devices, the sponsor submits the manufacturer's evidence (e.g., CE Marking Certificate) in the TGA Business Services (TBS) system for TGA review and acceptance.
(1)The registration process for Class I medical devices that do not require sterilization and do not have measuring functionality.
①The manufacturer prepares technical documentation and an Australian Declaration of Conformity.
②The agent applies for inclusion in the Australian Register of Therapeutic Goods (ARTG) through TGA eBS.
③The device is recognized and notified to the agent by ARTG and TGA, with the possibility of post-market review by ARTG.
④The agent prints the inclusion certificate from eBS.
⑤Monitoring of post-market products.
(2)Registration process for medical devices in other categories (i.e., Class I sterile, Class I measuring, Class IIa, Class IIb, Class III, AIMD class):
①Classify the product into Class I sterile, Class I with measuring functionality, Class IIa, Class IIb, Class III, AIMD.
②Evidence of conformity assessment from TGA or EU NB.
③The manufacturer prepares an Australian Declaration of Conformity.
④The agent submits the manufacturer's documentation to TGA.
⑤If the application is successful, the submission of the declared device will be included in the Australian Register of Therapeutic Goods (ARTG), and the agent can sell it in Australia. If it is not successful, the application will be withdrawn, and modifications are made to provide additional materials.
⑥Post-market surveillance of the product.
5. Application Submission: The sponsor submits a medical device application in the TBS system and pays the application fee. This application includes an "intended use" statement, classification, and a Global Medical Device Nomenclature (GMDN) code.
6. TGA Review: As a part of the Level 2 application review, the TGA will review various components of the design dossier. All Class III devices require a Level 2 application review, but only a small subset of Class IIb devices undergo this review.
7. Approval Granted: TGA will either approve or reject your application. If approved by TGA, you will receive an Australian Register of Therapeutic Goods (ARTG) listing number (which includes the certificate), and your listing will be included in the ARTG database on the TGA website.
8. Sales: You can now start selling your device in Australia. The registration will not expire as long as you do not make changes to the device that would invalidate the ARTG listing, the current CE marking certificate (if applicable) is on file with TGA, and annual ARTG listing fees are paid.