Medical devices CE Marking Process
I. CE Marking Introduction
The CE marking is a safety certification mark and is considered a passport for manufacturers to open and enter the European market.
‘CE marking of conformity’ or ‘CE marking’ of Medical Devices means a marking by which a manufacturer indicates that a device is in conformity with the applicable requirements set out in this Regulation and other applicable Union harmonisation legislation providing for its affixing.
II. Applicable Regulations
1. Medical Device Regulation (MDR) 2017/745/EU
2. In Vitro Diagnostic Regulation (IVDR) 2017/746
III. Categorization of Medical Devices
Medical devices can be categorized as Class I, Class IIa, Class IIb or Class III.
IV. System Assessment Requirements
1. Collect EU technical regulations and EU standards related to certified products;
2. The enterprise should be strictly in accordance with the above product standards for the organization of production, which means that the requirements of the above technical regulations and EU standards are implemented into the whole process of product design, development and manufacturing;
3. Enterprises must establish and maintain quality system in accordance with ISO9000+ISO13485 standards and obtain ISO9000+ISO13485 certification.
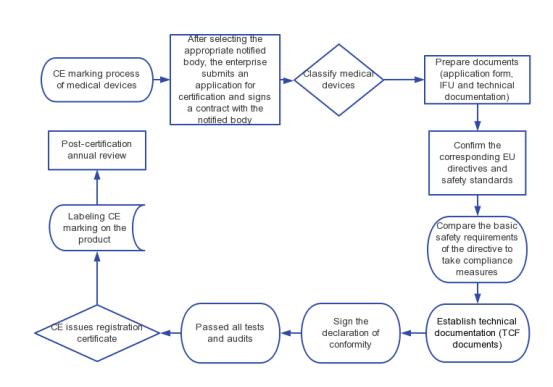
V. CE Marking Process
1.Choose a suitable notified body
2.Enterprises shall apply to the notified body for certification
3.Notified body signs a contract with the enterprise
4.Classify medical devices: primarily to confirm whether pre-market/post-market clinical data is required
5.Prepare registration information: e.g. product and model description, EC declaration of conformity, risk assessment, basic safety checklist, market feedback and analysis, instructions for use and labelling information.
6.Submit the quality management system documents, i.e. quality manual and procedure documents.
7.Establish technical documentation
8.Sign the declaration of conformity
9.Initial audit of quality system and technical documentation
10.Formal audit of quality system and technical documentation
11.Issue CE marking certificate
12.Annual review after certification
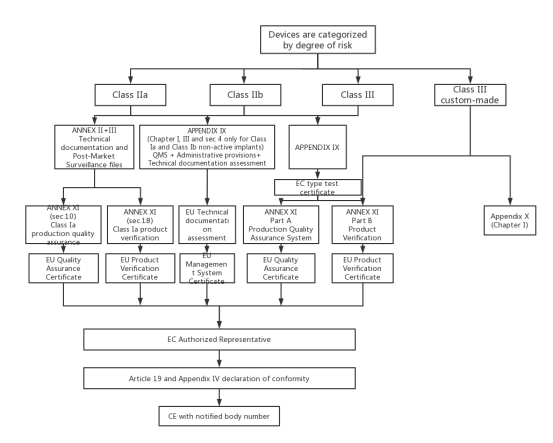
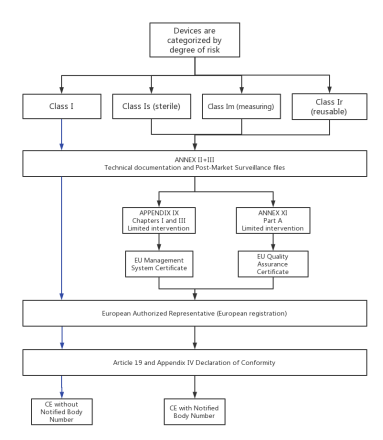
VI. Three Classes of Medical Devices CE Marking Steps:
1.CE marking steps of Class I medical devices
2.CE marking steps of Class II, III medical devices