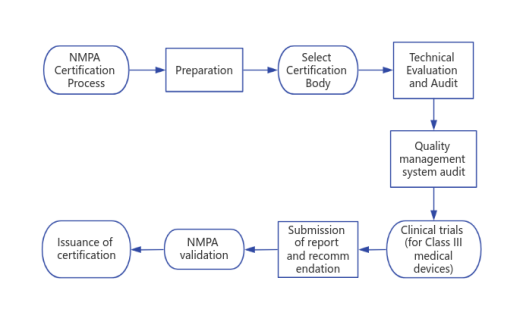
NMPA Certification Process
I. Introduction of NMPA
NMPA is the abbreviation of National Medical Products Administration of the State Drug Administration. NMPA is the only organisation responsible for the approval and registration of imported medical devices.
II. Regulations
Regulations for the Supervision and Administration of Medical Devices
Measures for the Administration of Registration and Filing of Medical Devices
Measures for the Administration of Registration and Filing of In Vitro Diagnostic Reagents
III. Classification
NMPA certification is divided into Class I, Class II and Class III medical device certification, which is classified according to the risk level of medical devices. Among them, Class I medical devices only need to be filed, while Class II and Class III medical devices need to undergo a more stringent certification process, including technical evaluation, quality management system audit and clinical trials (for Class III medical devices) and other steps.
IV. NMPA Certification
Medical device product registration declaration including "registration declaration", "change registration declaration" and "continuation of registration declaration".
“the Announcement on Issuing the Requirements for the Application Dossiers and the Format of Approval Certificates for Medical Devices Registration” (No. 121 of 2021) and “the Announcement on Issuing the Requirements for Registration Application Dossiers of In Vitro Diagnostic Reagents and Formats of Approval Documents” (No. 122 of 2021) gives the three registration declaration needs to be submitted to the information requirements.
Specific technical requirements for registration filings may also refer to applicable guidelines, review points.
V. System Assessment
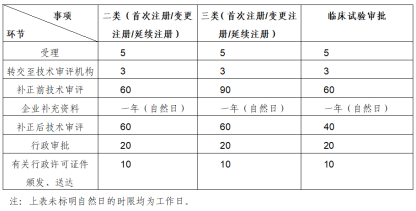
Time required for registration(Resource: NMPA)
NMPA classified products into three categories according to the complexity of the device, use and risk, Class I medical device approval usually takes 6-9 months will make a direct decision on a single submission will be sufficient for approval, while the approval of Class II medical device need 10-12 months; Class III medical device approval usually takes 12-18 months or even longer. Class II and Class III products need to be NMPA's subsidiary of the "Centre for Medical Device Evaluation (CMDE)" for technical review, and type testing by NMPA certified testers or testing laboratories.
VI. NMPA Medical Device Registration process
1. Preparation: Prepare relevant technical documents and application materials, including product technical specifications, clinical trial data (if applicable), and quality management system documents.
2. Select Certification Body: Select qualified NMPA accredited certification body, submit the application and pay the appropriate certification fees.
3. Technical Evaluation and Audit: The certification body conducts technical evaluation and audit of the application materials to ensure that the design and performance of the medical device comply with the relevant technical standards and regulatory requirements.
4. Quality management system audit: the certification body of the applicant's quality management system audit to ensure that the production process is effectively controlled, in line with quality management requirements.
5. Clinical trials (for Class III medical devices): If the application is for Class III medical devices, clinical trials may be required to verify their safety and effectiveness.
6. Submission of report and recommendation: The certification body will submit the audit results and certification recommendation to NMPA.
7. NMPA validation: NMPA validates the audit report and confirms whether the certification is in compliance with relevant regulations and policies.
8. Issuance of certification: If the certification is passed, the certification body will issue the applicant with the NMPA certification of the medical device, confirming that the medical device meets the quality and safety standards of the Chinese state and can be sold and used in the Chinese market.