Thailand Medical Device Registration Process
I. TFDA
TFDA is Thailand Food and Drug Administration, medical devices, food, drugs, supplements, products used for health care or other medical, anaesthetic and toxic substances entering Thailand need to be registered by Thailand Food and Drug Administration.
II. Regulations
1. Thailand Medical Device Act B.E.2551 and its amendments
2. Medical Device Act (No.2) BE 2562
All devices registered after 15 February 2021 shall comply with the new regulations and shall have their technical documents compiled in CSDT format. Devices approved under the current old regulations need to be replaced according to the new regulations.
III.Classification of Medical Devices
According to the risk of safety and effectiveness of medical devices, Thailand classifies medical devices into four categories: Class I, Class II, Class III and Class IV:
Class I: low risk - application route: self-declaration
Class II: low to medium risk - application route: CSDT
Class III: Medium to high risk - application route: CSDT
Class IV: High risk - Application route: CSDT
Low risk Class I devices must be listed prior to importation and sale in Thailand, while Class II and III devices must be notified and Class IV devices must have an approved licence before placing them on the Thai market.Classes II, III and IV devices require the submission of a technical dossier in accordance with the ASEAN CSDT format.Class I aseptic and measuring devices require the submission of a test report before these devices are placed on the market.Class I aseptic and measuring devices require the submission of a test report before these devices are placed on the market.
IV.TDFA Certification Information
1. Class I:
Business licence
Power of attorney from the designated operator
Equipment name and description, labelling, equipment specifications, manufacturing information or product owner details and instructions for use (IFU) (if applicable)
Foreign registration record (if applicable)
Sterilisation test report (for sterile devices)
Calibration test report (for measuring devices)
Declaration of Conformity (DoC) from manufacturer or product owner
Letter of Authorisation (LOA)
2. Class II-IV:
TFDA has issued transitional regulations allowing manufacturers and importers until 15 February 2024 to follow the simplified requirements. However, after the deadline, technical dossiers submitted for Class II-IV devices need to meet the full CSDT requirements. The CSDT format requirements are described in Annex 4 of the ASEAN Medical Device Directive (AMDD).
Operating Licence
Power of Attorney from the Designated Operator
Device name and description, labelling, manufacturing information, product owner details and executive summary
Waste disposal procedures (if applicable)
Quality management system certification (ISO/GMP)
Manufacturer's or Product Owner's Declaration of Conformity (DoC)
Letter of Authorisation (LOA)
V. System Assessment
1. The applicant must have a comprehensive product storage plan, including instructions to place the medical device storage location of the internal floor plan, and detailed description of the space allocation of the storage space, storage facilities installed, and programmes to reduce the stock of personal and public health.
2. Medical devices need to have proof of registration as a manufactured medical device before entering Thailand. It is very difficult for non-Thai manufactured medical devices to obtain Thai TFDA approval solely on the basis of medical device registrations in other countries. It is possible to apply for Thai medical device registration for a product that does not have a medical device registration certificate in the country of origin if there is a valid reason to do so, e.g., the product is intended for use by a population other than the country of origin.
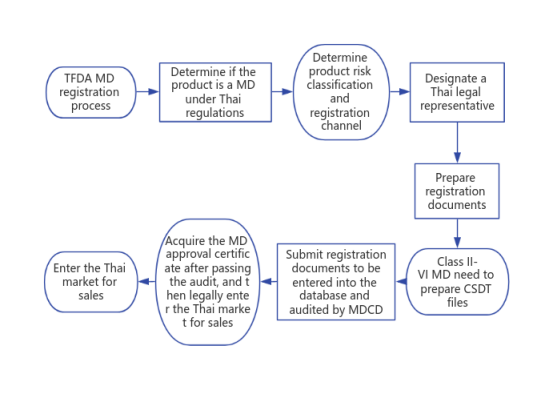
VI. TFDA medical device registration process
1. Determine whether the product is a medical device under Thai regulations as well as the product risk classification and registration channel;
2. Designate a Thai legal representative;
3. Prepare registration documents, including free sale certificate and system to prove compliance documents;
4. Class II-VI medical devices need to prepare CSDT files;
5. Submit registration documents to be entered into the database and audited by Medical Device Control Department (MDCD);
6. Acquire the medical device approval certificate after passing the audit, and then legally enter the Thai market for sales.