Singapore Medical Device Registration Process
I. Introduction of HSA
In Singapore, medical devices are regulated by the Medical Devices Division of the Health Sciences Authority (HSA). Singapore is one of the Association of Southeast Asian Nations (ASEAN) member countries and its regulatory system is based on the Health Products Act 2007 and the Health Products (Medical Devices) Regulations 2010.
Before importing, distributing or selling any medical devices, cosmetics, food, healthcare products in Singapore, they must be registered with the HSA. Only licensees or registrants of locally registered companies can submit medical devices for registration. The Singapore HSA follows the GHTF guidelines for classification of medical devices.
II. Regulations
1、Health Products Act 2007.
2、Health Products (Medical.Devices) Regulations 2010.
3、GN-15-R9 guidance on medical-device product registration
III. Classification of Medical Devices
Medical devices are categorized into four risk classes in Singapore, and manufacturers are required to classify each medical device according to its intended use. class A has the lowest risk class, while class D has the highest risk class.
Classification | Level | Example |
A | Low | Tongue depressors, glass liquid thermometers, medical masks, stethoscopes, walkers... |
B | Medium-low | Hypodermic needles, anesthesia breathing circuits, hearing aids... |
C | Medium-high | Ventilators, orthopedic implants, infant incubators, blood collection bags, defibrillators... |
D | High | Cardiac pacemakers, implantable defibrillators, implantable infusion pumps, heart valves, intrauterine contraceptive devices, nerve conduits... |
IV. HSA Certification Information
The Health Sciences Authority of Singapore (HSA) registration application submission information or technical documents are based on the ASEAN CSDT (Common Submission Format) file format. The information requested in the submission depends on the classification of your medical device and the assessment pathway chosen. Documentation from the EU Technical File is mostly available to fulfill many of the supporting documentation requirements.
QMS: proof of QMS is required
Labeling: Labeling must be in English; at the same time, HSA is phasing in UDI, starting with high-risk Class D
Standards: HAS recognizes various international and regional national standards
Clinical data: Each medical device requires clinical data suitable for the use and classification of the device, HSA can accept clinical data from other countries
V. System Assessment
Statutory manufacturers who do not have a place of business in Singapore must appoint a local representative, the registrant, who can act as a legal person, organization or company, to submit an application for registration to the HSA. The registrant must have a Singapore company registered with HSA and hold an ACRA certificate. The registrant does not need a license from HSA but must be registered in the Medical Device Information Communication System.
All medical devices must be submitted by the registrant, who makes the submission and is responsible for some post-market activities.
The registrant is also required to supervise and control the manufacturer's list of authorized importers in Singapore, and if the registrant is an independent third party, the registrant is required to authorize the distributor or importer to complete the closed loop.
Multiple registrants can be registered for the same equipment. If the relationship between the company and the previous registrant breaks down, the registration must be done again unless the previous registrant is willing to transfer the certificate.
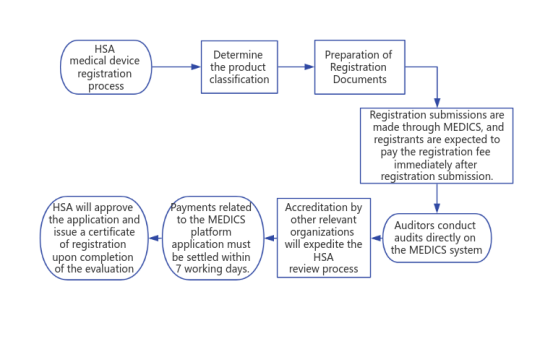
VI. HSA medical device registration process
1、Determine the product classification
Determine the classification of registered products according to the classification rules of "gn-14-r3 guidance on the risk classification of in vitro diagnostic md" issued by HSA.
Class A devices are exempted from registration audit, but such medical devices should comply with all relevant medical device safety and performance requirements in the regulations before they are placed on the Singapore market. The local representative/distributor is required to list the product in the Health Sciences Authority (HSA) Class A medical device database through MEDICS.
Class B, Class C and Class D products need to be registered.
2、Preparation of Registration Documents
Singapore's MD and IVD product registration information in addition to administrative documents such as Singapore's authorized representative of the Establishment Licensing and letter of authorisation in addition to technical information in accordance with the ASEAN CSDT template for preparation.
ASEAN CSDT format checklist:
(1) Executive Summary
(2) List of Basic Principles
(3) Declaration of Conformity
(4) Equipment description
(5) Details of design verification and validation documents.
① Complete reports of preclinical studies, such as physical test data, biocompatibility studies, animal studies, and software verification and validation studies
② Measurement requirements (if applicable)
③ Sterilization validation (if applicable)
④ Shelf life studies and expected useful life
(6) Clinical evaluation reports, including publications and full reports of cited studies
(7) Device labeling
(8) Risk analysis
(9) Name and address of manufacturing and sterilization sites
(10) Proof of at least one of the following quality management systems.
① ISO 13485 quality management system certificate
② Proof of compliance with the U.S. FDA quality system QS820
③ GMP certificate in compliance with the Japanese Ministry of Health, Labor and Welfare regulations
(11) Production process - process flow diagrams
3、Registration submission
All medical devices and in vitro diagnostic reagents registration must be submitted by the registrant through the Medical Device Information and Communication System (MEDICS) online.
The registrant shall pay the registration fee immediately after the registration is submitted.
After HSA receives the registration application, the auditor will carry out the audit work directly on the MEDICS system and will respond to the corresponding information through the MEDICS system, such as requesting further additional documents or instructions. All communications are made through the MEDICS system, which automatically sends an email message to the registrant (the authorized representative in Singapore).
For registration applications that meet the requirements, HSA will approve the application and issue a certificate of registration upon completion of the assessment and notify the registrant. For those that do not meet the requirements, HSA will reject the registration application.
4. Accelerated Approval Process
If the medical device is certified by the relevant organizations in the United States (FDA), Europe (EU NB), Australia (TGA), Japan (MHLW) or Canada (HC), it will speed up the review process of the Health Sciences Authority (HSA).
5. Official Registration Fees
All submitted medical device applications, or changes at a later stage will require a corresponding fee.
The HSA will automatically generate a fee invoice upon receipt of the application on the MEDICS platform. The payment must be settled within 7 working days. If, for whatever reason, payment is not made within the deadline, the application will be considered overdue and will need to be resubmitted.